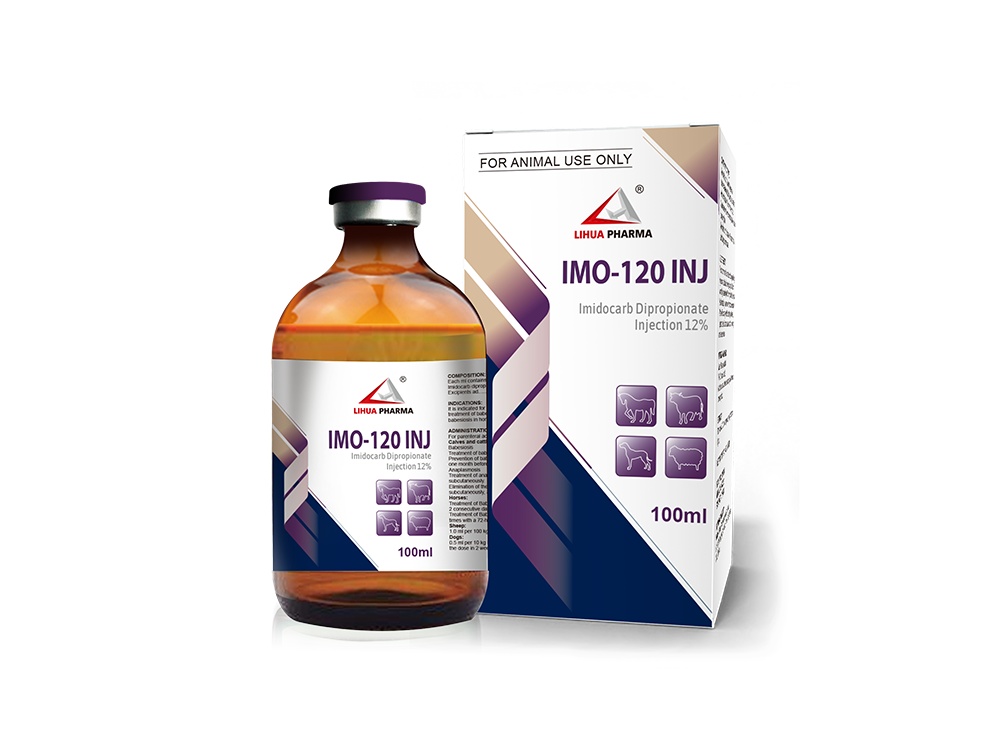
Imidocarb Dipropionate Injection 12%
Indications
It is indicated for treatment and prophylaxis of babesiosis in cattle, for treatment of babesiosis and anaplasmosis in sheep, for treatment of babesiosis in horses and dogs and for treatment of anaplasmosis in cattle.
Contraindications
Administration to animals exposed to cholinesterase-inhibiting drugs or pesticides.
Administration via the intravenous route.
Administration to ewes producing milk for human consumption.
Administration to animals with impaired renal and/or hepatic functions.
Side Effects
Most common adverse effects include pain during injections and mild cholinergic signs (salivation, vomitting, nasal drip). Cholinergic side effects may be alleviated by treatment with atropine sulphate. Other effects may include panting, diarrhoea, injection site inflammation, lacrimation, sweating and restlessness.
Administration and Dosage
For parenteral administration.
Calves and cattle:
Babesiosis
Treatment of babesiosis: 1.0 ml per 100 kg body weight, subcutaneously.
Prevention of babesiosis: 2.5 ml per 100 kg body weight, subcutaneously, one month before exposure.
Anaplasmosis
Treatment of anaplasmosis: 2.5 ml per 100 kg bodyweight, subcutaneously.
Elimination of the carrier state: 4.0 ml per 100 kg bodyweight, subcutaneously, administered twice with a 14-day interval.
Horses:
Treatment of Babesia caballi: 2.0 ml per 100 kg body weight, once daily for 2 consecutive days, intramuscularly.
Treatment of Babesia equi: 3.5 ml per 100 kg body weight, administered 4 times with a 72-hour interval.
Sheep:
1.0 ml per 100 kg body weight, intramuscularly.
Dogs:
0.5 ml per 10 kg body weight, subcutaneously or intramuscularly. Repeat the dose in 2 weeks, for a total of 2 treatments.
Withdrawal Period
Meat: 90 days (cattle).
Milk: 21 days (cattle).
Not to be used in sheep producing milk for human consumption.
Storage
Store below 30℃, in a dry place, and protect from light.
For Veterinary Use Only
You may also be interested